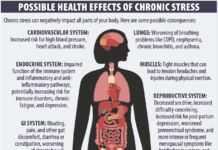
As 2023 draws to a close, let’s review and update some of the biggest health and nutrition stories of the year.
Concerns About Sugar Substitutes. Sugar substitutes have been around for decades, and concerns about their safety have come and gone. The past year saw several noteworthy storeis regarding these popular additives.

Cutting back on all kinds of sweeteners and sweet-tasting foods and beverages is a better strategy for health than switching sweeteners. Enjoy reasonable portions of sweets as treats.
Sugar alcohols are naturally occurring sweet compounds that have less calories than sugar, do not cause tooth decay, and have low impact on blood sugar levels. They are extracted from natural sources or synthesized in a lab and used in products such as sugar-free candies, cookies, ice cream, beverages and chewing gum.
In February 2023, a study reported associations between higher levels of the sugar alcohol erythritol in the blood and higher risk of heart attack and stroke. (See page 2 of our July 2023 issue for more information.) These findings have yet to be confirmed. We will continue to follow this story.
Artificial sweeteners are chemically synthesized, highly sweet substances that are used as sugar alternatives in processed foods such as sweet drinks, frozen desserts, candies, chewing gum, and puddings—particularly those labeled “diet” or “zero-calorie”. Unlike sugar, artificial sweeteners contribute little or no calories to foods and beverages, so they are appealing sugar substitutes for those trying to reduce their added sugar intake, loss body weight, and manage blood glucose levels. However, when it comes to whether or not artificial sweeteners actually help with weight control, the research is inconclusive. Some studies that follow people for long periods of time have concluded that consuming artificially sweetened beverages instead of sugar-sweetened beverages reduces calorie intake and helps slow weight gain or maintain weight loss, but other studies show no benefit. (One reason some studies show no benefit may be because people who choose artificially sweetened “diet” beverages may do so because they are struggling with unwanted weight gain. Including a high proportion of these individuals in a study would bias the results. It is also theorized that artificial sweeteners may stimulate appetite and sweet preference, resulting in higher caloric intake from other sources.)
Given that higher consumption of added sugars is associated with higher risk of chronic diseases like cardiovascular disease, obesity, type 2 diabetes, and non-alcoholic fatty liver disease, whereas artificial sweeteners tend not to be associated with this risk, it is tempting to see artificially sweetened foods as a way to keep enjoying sweets while avoiding the health dangers posed by added sugars. While some studies suggest a lower risk of type 2 diabetes when artificially sweetened beverages are used instead of sugar-sweetened options, some evidence suggests that consumption of artificially sweetened beverages is actually associated with higher cardiometabolic risk, including high blood pressure, metabolic syndrome, type 2 diabetes, heart attacks and stroke. However, it is important to note that the concept of reverse causation likely contributes to the contradictory evidence: people who already have poor cardiometabolic health may be more likely to choose artificially sweetened beverages as a strategy to control blood sugar. Furthermore, research results may vary based on whether study participants used artificially sweetened beverages to replace sugar sweetened beverages or if they drank them instead of choices like water or seltzer.
In May of 2023, the World Health Organization (WHO) released a guideline recommending against the use of artificial sweeteners to control body weight and reduce the risk of chronic disease. Two months later they released a statement classifying the common artificial sweetener aspartame as “possibly carcinogenic.” It’s important to understand that this determination was based on limited evidence in humans. In fact, the WHO did not find enough evidence to warrant changing the recommended upper daily intake limit for aspartame—40 milligrams per kilogram of body weight per day. To reach this upper limit, a 150-pound person would need to consume the equivalent of 11 12-ounce cans of diet soda a day. The report concluded more research is needed to understand whether aspartame truly increases cancer risk in humans. In general, available evidence on the safety of artificial sweeteners has limitations, and long-term randomized controlled trials are lacking. If there is evidence that an artificial sweetener causes cancer, the U.S. Food and Drug Administration is obligated to ban its use.
While consumption of sugar alternatives can be helpful in reducing high intake of added sugars, an overall reduction in sugar and sugar alternatives is probably the best option for long-term health.
A New Look at Body Mass Index (BMI). Body Mass Index (BMI) is a ratio of weight to height. It has been used to group people as underweight, “normal” weight, overweight, or obese. While it is a useful public health tool, BMI’s ability to predict health risks on an individual level has recently been questioned.
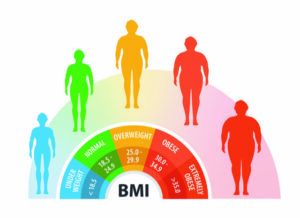
Images © CollageM | Getty Images
Body mass index (BMI) alone should not be used as an indicator of disease risk. It is not accurate for everyone.
BMI (calculated by dividing weight in kilograms by height in meters squared) is often used to predict one’s risk of developing a chronic disease such as heart disease or type 2 diabetes. However, in our October 2023 issue we summarized a study that concluded a BMI from 22.5 to 29.9 kg/m2 was not associated with higher risk of death, especially in older adults. [Editor’s Note: In that NewsBite on page 1 of the October issue, we wrongly stated this BMI range as “overweight.” The standard definition of “overweight” is 25.0 to 29.9 kg/m2. A BMI of 18.5 to 24.9 kg/m2 is considered “normal weight.” We apologize for any confusion this may have caused.)
In June of 2023, the American Medical Association (AMA) issued a new policy stating that BMI alone should not be used to draw conclusions about chronic disease risk. This policy was based partly on the new data, but also because BMI does not account for differences across racial/ethnic groups, sexes, genders, and body composition. This makes it an imperfect way to estimate body fatness. (BMI has always been problematic for people with a high percentage of muscle.)
You and your healthcare provider should not ignore your BMI, but it should be used in conjunction with other measures of risk, such as waist circumference, blood pressure, and blood levels of cholesterol, triglyceride, and glucose (blood sugar) when determining risk for conditions like cardiovascular disease and type 2 diabetes.
Treatments for Alzheimer’s Disease. Alzheimer’s disease is the leading cause of dementia. While there is currently no cure for this devastating disease, there are worldwide efforts underway to develop medications that prevent its development or slow its progression. In July 2023, the U.S. Food and Drug Administration (FDA) approved lecanemab (marketed as Leqembi) for use in the early stages of Alzheimer’s disease.
Like the previously approved aducanumab (marketed as Aduhelm), lecanemab uses amyloid-targeting approaches to slow the progression of Alzheimer’s disease. Amyloid protein is involved in the formation of plaque in the brain, which contributes to decline of memory and function. By targeting this protein, these medications slow plaque formation. This does not cure the disease, but may lead to slower cognitive and functional decline and extend the time people with this disease can live independently. Both medications were approved to treat people with early symptoms of Alzheimer’s disease, including those living with mild cognitive impairment.
These drugs may have side effects, including allergic reactions, infusion-related reactions (such as flushing, rash, fever, or chills), headaches, nausea, and falls. Another potential side effect is amyloid-related imaging abnormalities (ARIA)—a swelling in the brain that is usually temporary and typically asymptomatic, but can, in some cases, be serious. There is a genetic risk factor that may put some individuals at increased risk of ARIA, so the FDA suggests genetic testing prior to using these amyloid-targeting drugs.
➧ Cut Back on Sweets of All Kinds. Drink and eat fewer sweet treats, even if they are sweetened with sugar substitutes.
➧ Look Beyond BMI. A high body mass index (BMI) is often used as a criterion to assess risk for developing chronic diseases like cardiovascular disease and type 2 diabetes, but it may not be the best indicator.
➧ Follow the Fight Against Dementia. The first drugs that treat an underlying cause of the most common form of dementia—Alzheimer’s disease—have been approved. Talk to your healthcare provider about these options if you or someone you love are in the early stages of dementia.
There are other medications on the market that can help reduce or stabilize symptoms of Alzheimer’s disease progression, such as memory loss and confusion, but lecanemab and aducanumab are the first that impact pary of the underlying biology of the disease.
The recent approval of lecanemab demonstrates the meaningful progress ids being made in the scientific understanding of Alzheimer’s disease. As with all medications, it’s important to work with a physician to develop a treatment plan that is right for you.