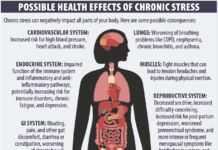
In what may prove a landmark ruling for food and beverage health claims, a US appeals court largely upheld a Federal Trade Commission order requiring proof of promises made for a popular pomegranate-juice drink. In its original 2010 order, the FTC had challenged magazine advertisements for POM Wonderful that claimed the beverage combats heart disease and other ailments. The appeals-court ruling overturned the agency’s requirement that two human clinical trials back up such claims in all cases as “too onerous.” But the three-judge panel agreed that the ads were misleading and that at least one randomized clinical trial could be required to substantiated any claims of treating or preventing disease.