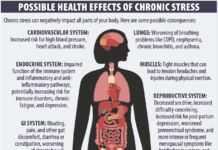
The FDA and the Federal Trade Commission (FTC) recently issued warnings to 10 companies regarding fraudulent marketing claims that their dietary supplements prevent, treat, or cure diabetes.
The companies made claims that certain products could lower blood glucose levels, reduce hemoglobin A1c, or impact health in other ways. Claims that a product can cure, mitigate, treat, or prevent disease are only allowed on drugs that have been proven safe and effective. Regulations on dietary supplements do not allow them to be marketed to cure or treat disease.
Products of concern include: Pharmaganics LLC’s Diabetes Doctor Pre-Diabetes and Diabetes Doctor Blood Sugar 24 Hour; Lysulin brand Weight Loss Shake, Diabetes and Prediabetes Chewables, Diabetes and Prediabetes Liquid, Diabetes and Prediabetes Capsules, and Diabetes and Prediabetes Powder; and Phytag Labs’ GlucoType2.
To control blood glucose levels, focus on adhering to a healthy dietary pattern and engaging in regular physical activity.